MAIN INNOVATIONS
Redox flow batteries are designed to work at temperature of up to 40°C, however, the battery generates heat. Under certain circumstances, a cooling system is required to avoid electrolyte degradation or battery malfunction. Cooling requires energy and reduces the battery global efficiency. That is why our battery will be developed to work at higher temperatures.
A BATTERY MADE OF ORGANIC ELECTROLYTES AND INFLATABLE TANKS
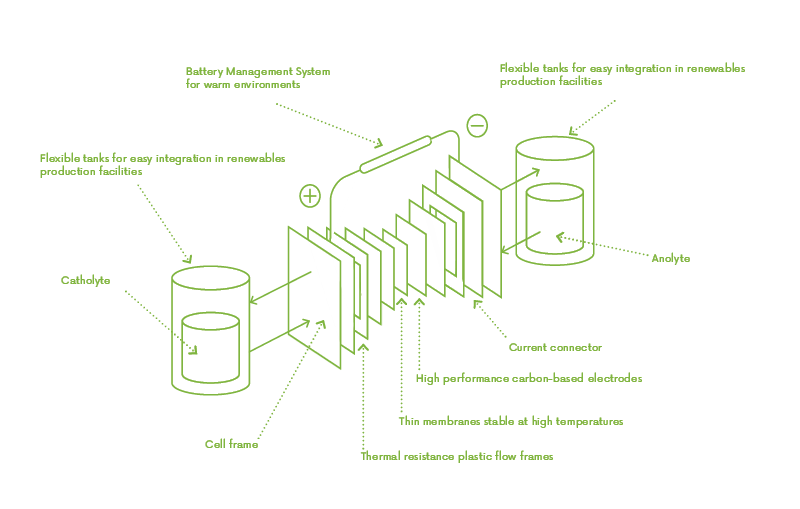
Redox flow batteries are made up of two tanks filled with electrolyte fluids.
When circulated through two half-cells separated by a membrane, the electrochemical reactions for charging or discharging takes place.
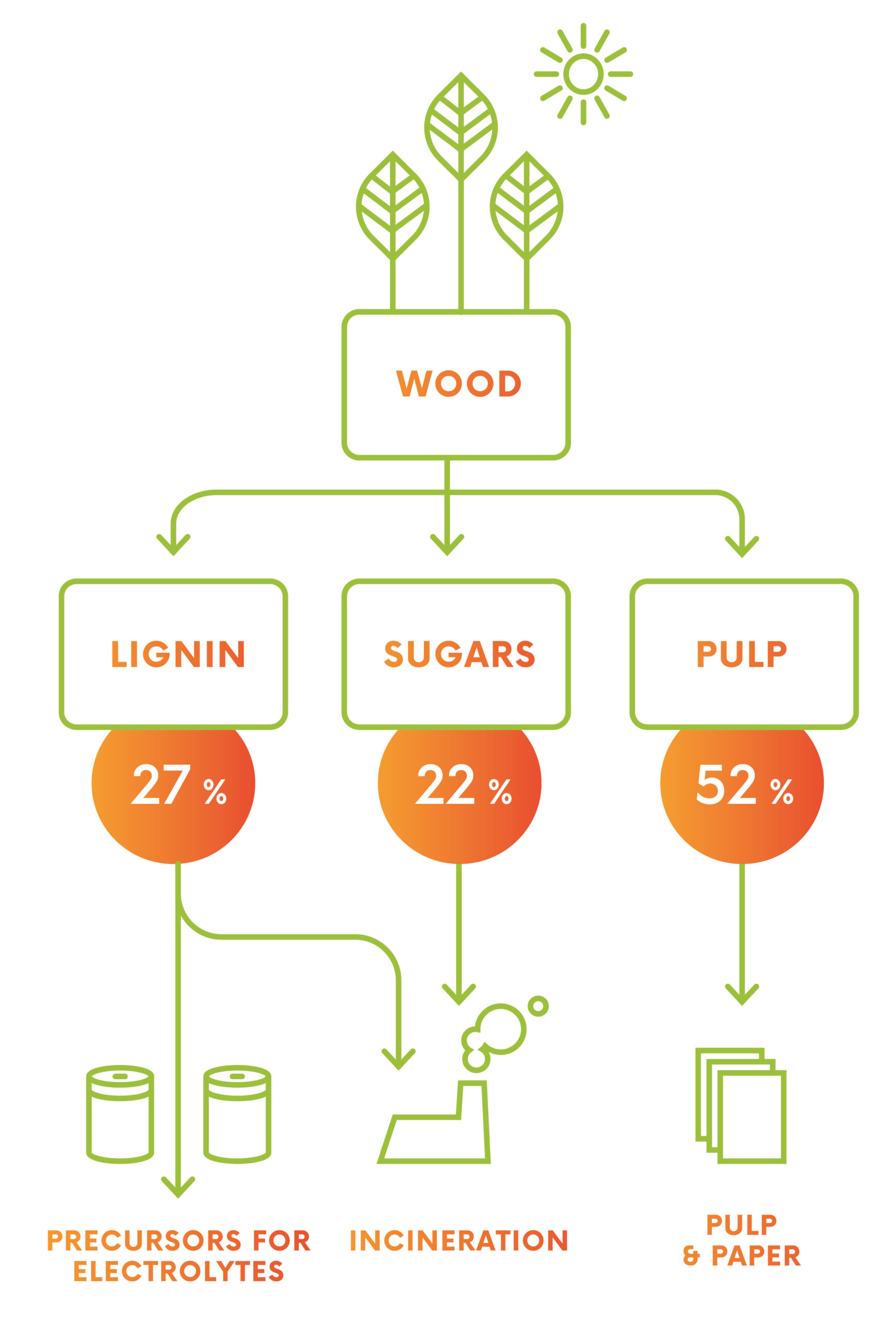
Unlike other redox flow batteries, our organic RFB – we call it Organic Flow Battery – will use electrolytes that can be made out of intermediate products which can be obtained from lignin.
Lignin is a natural and renewable raw material and is available in sufficient amounts from existing pulp production.
In addition, our tanks will be double-wall flexible containers with unlimited and modular size which will permit upscaling the battery’s capacity.
Basic principle of a redox flow battery